The REACT (REAl world effeCtiveness of allergy immunoTherapy) study
REACT is a large, retrospective real world effectiveness study, applying high scientific standards outlined by organisations such as the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) or the Respiratory Effectiveness Group (REG). The aim of REACT is to assess the long-term effectiveness of AIT in the treatment of allergic rhinitis and asthma.
The REACT study was designed applying good practice principles in RWE:
- All analyses and outcomes were pre-specified
- Measures were taken to avoid bias and confounding, in particular propensity score matching (PSM) was used to ensure comparable groups
- The study was registered on clinicaltrials.gov (Clinicaltrials.gov Identifier: NCT04125888) for transparency
Learn more on good practice principles of RWE or on guidelines on how to do RWE.
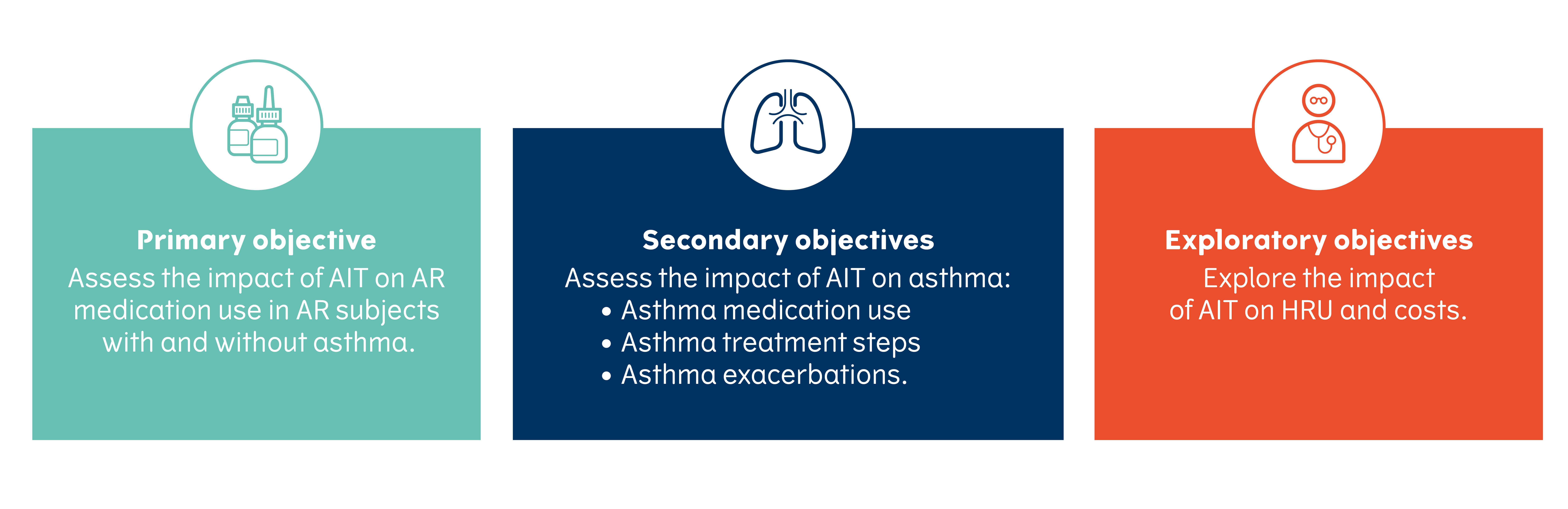
Study objectives were pre-specified
Primary objective of the study was to assess the impact of AIT on AR medication use in allergic rhinitis patients with or without asthma.
Secondary objectives of the study were:
- To assess the impact of AIT on asthma medication use in allergic rhinitis patients with pre-existing asthma
- To assess the impact of AIT on asthma disease severity, by looking at asthma treatment steps and asthma exacerbations.
Exploratory objectives of the study were to explore the impact of AIT on health care resource utilization (HRU) and health care costs. In addition, novel benefits were explored, such as the impact of AIT on pneumonia.
Real world data (RWD) was obtained from a large database representative of the statutory insured German population
For this retrospective observational, propensity score matched cohort study, anonymized claims data during 2007 to 2017 from a German health insurance fund database (Betriebkrankenkasse (BKK)) was used. The BKK database contains multiple data sources such as:
- General data (e.g. age and gender)
- Treatment data (e.g. prescription dates)
- Medical aids (e.g. ID codes)
- Sick leaves (e.g. diagnosis codes and duration)
- In- and outpatient data (e.g. duration of stay and costs)
With anonymous data of 5,9 million people across Germany, this dataset is representative of the statutory insured population, which is 90% of the total German population
A cohort of allergic rhinitis patients with AIT was identified and matched to allergic rhinitis patients not treated with AIT
Patients were eligible for the study if they met specific inclusion criteria. A general criterion was that data needed to be available at least 12 months before and after AIT initiation to establish a baseline and follow-up period. The date of the first filled AIT prescription is set as index date.
Patients eligible for the AIT cohort had:
- Two or more AIT prescriptions during the first year of treatment
- A confirmed AR diagnosis
- No venom AIT (e.g. bee or wasp allergy)
Patients eligible for the control cohort had:
- No AIT prescription one year prior to the index date
- A confirmed AR diagnosis
- No venom AIT (e.g. bee or wasp allergy)
The cohorts were build as follows:
Step 1: AR patients with or without asthma were identified in the BKK database.
Step 2: AR patients treated with AIT were matched to similar controls not treated with AIT
Step 3: Within each cohort, we distinguished patient with and without pre-existing asthma. The inclusion criteria for pre-existing asthma was an asthma diagnosis code or two prescriptions of inhaled corticosteroids (ICS) or short-acting beta agonists (SABA) in the year prior to starting AIT.
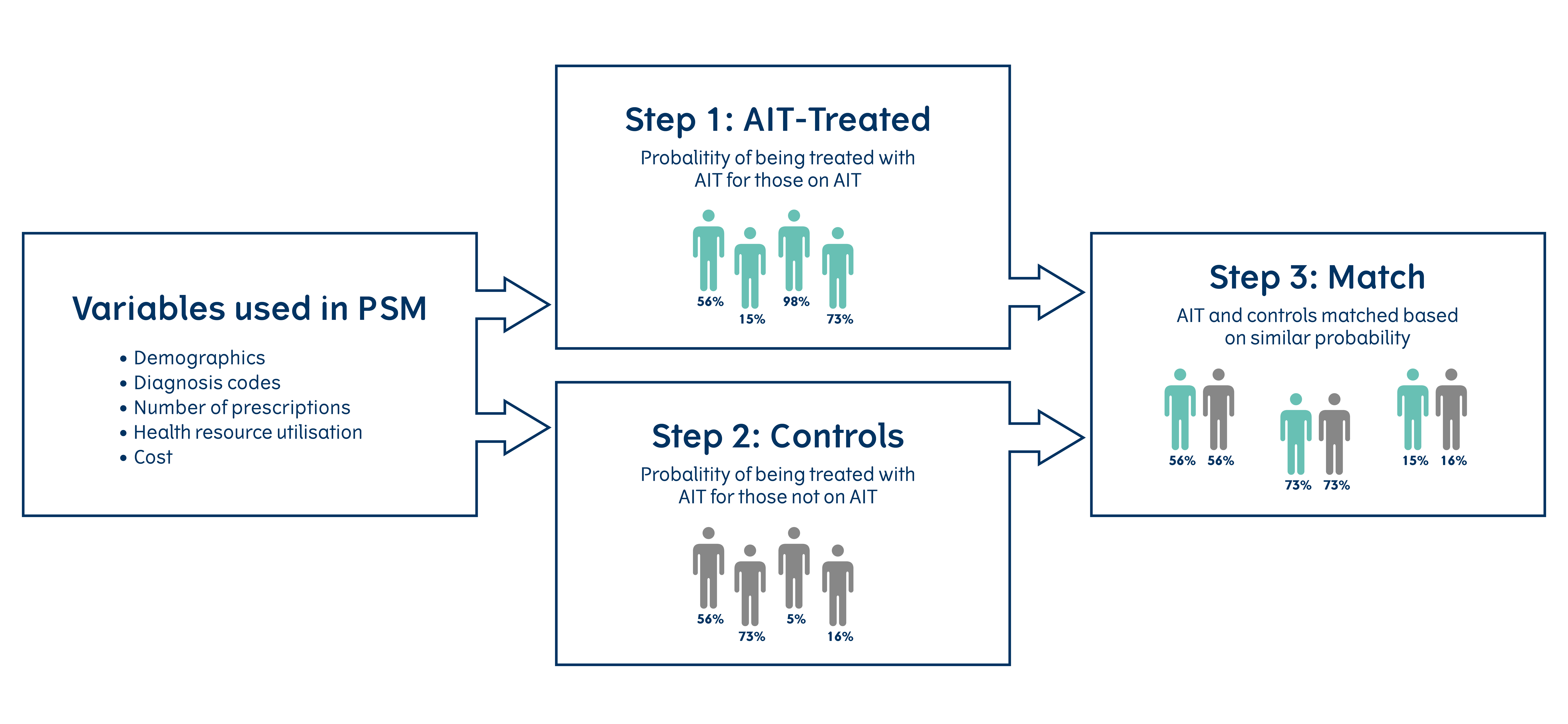
Propensity score matching was used to ensure comparable groups and mitigate confounding
As explained in ‘Pitfalls of RWE‘, the lack of randomisation can pose a risk of bias and confounding in RWE studies. In this study, this risk is mitigated by matching patients using propensity scores. The propensity score is the probability of being assigned treatment, based on patients’ characteristics. The propensity score allows one to design and analyse real world studies in a way that mimics some characteristics of a randomized controlled trial. The propensity score is a balancing score, ensuring comparable groups at baseline
How does propensity score matching work?
Step 1: The probability of being treated with AIT is calculated based on the characteristics of the AIT patient.
Step 2: The probability of being treated with AIT in controls who are not treated with AIT is calculated.
Step 3: Matching subjects from each group that have a similar score (similar probability of being treated). Subjects without a strong match are excluded from the study.
In this study, the cohorts were locked before outcomes were analysed, to avoid result-driven modifications to the PSM.
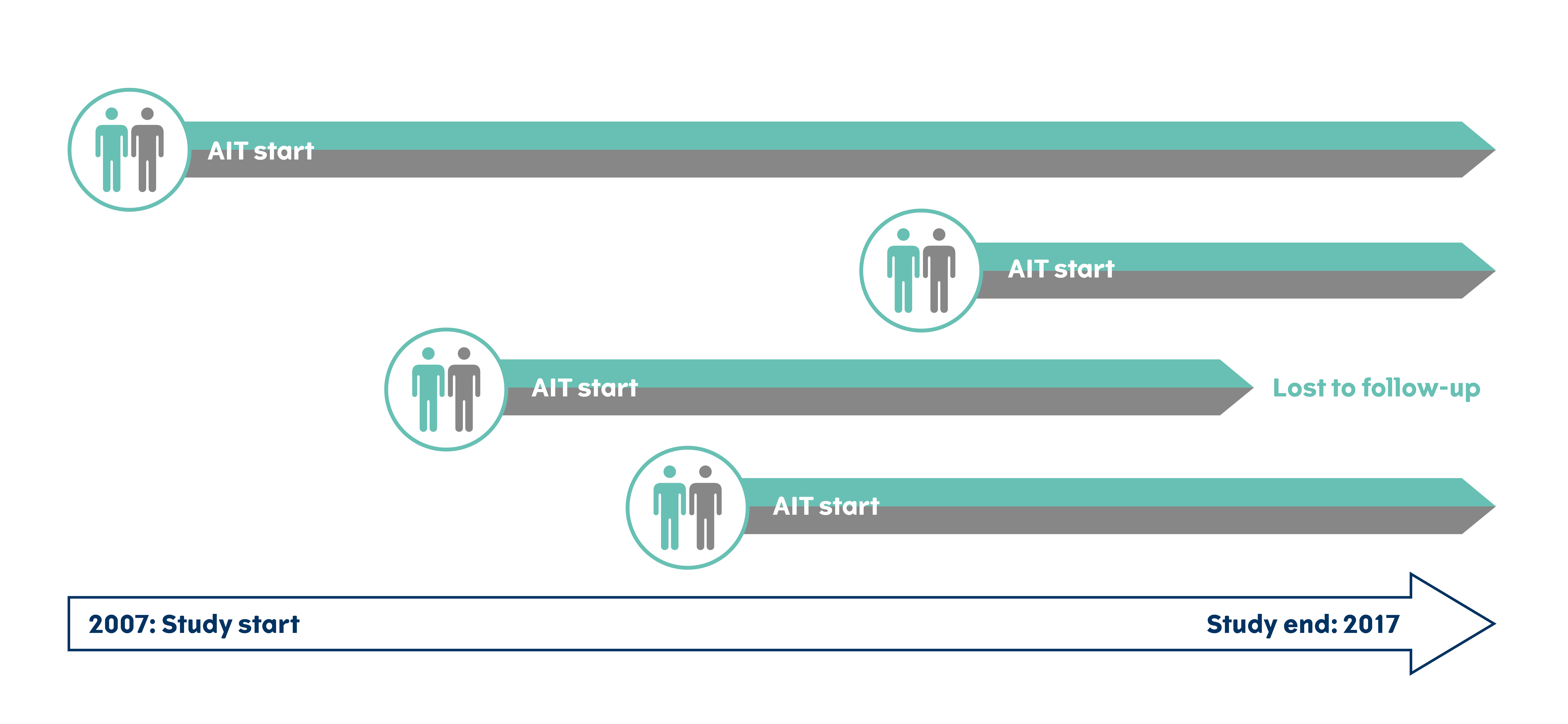
AIT patients and controls were followed for 9 years
The observation period of the study lasted from 1st of January 2007 until 31st of December 2017. Every matched pair (patient treated with AIT and the matched control) was followed from the start date of the AIT patient’s initiation until end of 2017 or until lost to follow-up. The earliest possible start date was 1st of January 2008, as a 12 months enrollment period was needed to establish a baseline. Thereafter, subjects were continuously enrolled with the latest possible start date being the 31st of December 2016, as a 12 months enrollment after start date was needed as a minimum to analyse outcomes. Therefore, patients could at a minimum be followed for 1 year and as a maximum for 9 years. When an AIT treated patient or the control was lost to follow-up, the matched counterpart was also lost. Loss to follow-up occurs as patients leave the database due to change in insurance or death.